Enhancer of zeste homolog 2 (EZH2), the catalytic subunit of the polycomb repressive complex 2 (PRC2), is a highly conserved histone methyltransferase that targets lysine-27 of histone H3. EZH2 is one of the key determinants of cellular sensitivity to DNA damage, and therefore an abnormal accumulation of EZH2 represses tumor suppressors, including genes related to cell cycle inhibition, apoptosis, senescence, and differentiation. Similar to EZH2, EZH1 is part of the PRC2 complex and shares target genes.
Acute myeloid leukemia (AML) is a heterogeneous disease associated with high mortality, dependent on molecular subtype and therapy. Bcl-2 family proteins have been established as key mediators of apoptosis in AML with venetoclax, a small molecule BH3 mimetic, selectively inhibiting Bcl-2. Combinatorial therapy of venetoclax and hypomethylating agents (Ven/HMA) has revolutionized the treatment of elderly patients with AML, yielding response rates over 70%, but overall survival remains short. Since PRC2 is required for AML cell survival (Basheer et al., 2019; Neff et al., 2012), targeting both EZH1 and EZH2 (EZH1/2) concomitantly could be efficient in disrupting oncogenic signals. A recent study using a murine AML model demonstrated that genetic deletion of EZH1/2 depleted quiescent leukemic stem cell (LSC) and prolonged survival of leukemia bearing mice (Fujita at al., 2018). AML LSC are located in BM niches and are mainly non-cycling. Thus, overcoming LSC dormancy as well as LSC mobilization from the BM may be critical steps that improve long-term survival of patients with AML. Here, we hypothesized that EZH1/2 is required to regulate LSC dormancy and increase efficacy of Ven/HMA therapy in AML. To test this hypothesis, we utilized pharmacological inhibition of EZH1/2 by a potent and selective EZH1/2 dual inhibitor, DS-3201 (valemetostat; Daiichi Sankyo, Inc.). Granulocyte colony-stimulating factor (G-SCF) has been shown to not only recruit leukemia cells into cell cycle (Andreeff et al, 1990), but also disrupt AML-stromal interactions for stem cell mobilization. Clinically, G-CSF is a critical component of the FLAG-Ida protocol (Petti et al, 1997). Therefore, we hypothesized that G-CSF will also recruit quiescent LSC into cycle, and the alternative mechanism may act synergistically with EZH1/2 inhibition.
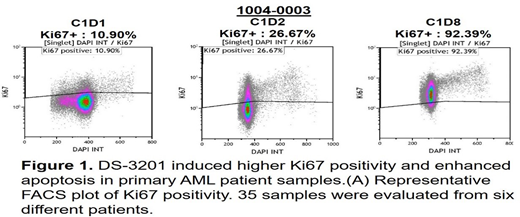
To assess if inhibition of EZH1/2 results in recruitment of LSC into the cell cycle and subsequently improves efficacy of Ven/HMA in AML in vitro, we first confirmed the nuclear expression of EZH1/2 in human AML cell lines. All cell lines expressed EZH1/2, although at different levels. These cell lines with various genetic backgrounds were treated with DS-3201 or G-CSF 24 hours prior to their exposure of Ven/HMA. A dual inhibitor of EZH1/2, or G-CSF, in combination with Ven/HMA significantly amplified AML cell death compared to Ven/HMA alone treated counterparts. In a clinical phase 1 trial of DS-3201 in AML, EZH1/2 inhibition promoted cell cycle progression. The proliferative fraction of leukemic cells was assessed by measuring Ki67/DNA in sequential samples obtained from DS-3201 treated patients. Baseline Ki67 positivity of LSC was 11.20%, and the maximized average increased to 59.3% and to over 90% in one patient (Fig 1), validating the concept that EZH1/2 inhibition can indeed recruit leukemia stem cells effectively into cell cycle. Further, a primary sample from a R/R AML patient (resistant to DNR/Ara-C 7+3, Ven/HMA, and Ipilimumab/nivolumab) was ex vivo primed for 5 days with combination of DS-3201 and G-CSF, then exposed to AraC/DNR based treatment. Concomitant treatment of G-CSF and EZH1/2 inhibition increased LSC proliferation and subsequently enhanced AraC/DNR induced apoptosis. Conclusion: we here demonstrate that pharmacological inhibition of EZH1/2 in a clinical trial with DS-3201 resulted in massive recruitment of quiescent AML LSC into the cell cycle. In vitro, EZH1/2 inhibition and G-CSF both enhanced Ven/HMA-induced leukemia cell apoptosis. Experiments are ongoing to test the hypothesis that EZH1/2 inhibition combined with G-CSF receptor activation may sensitize resistant primary AML LSC to apoptosis induction by chemotherapy and/or Bcl-2 inhibition. If successful, this concept could further enhance the activity of FLAG-Ida chemotherapy, which was developed a generation ago along the same concept, and of BH-3 mimetic, apoptosis targeting therapies.
Dos Santos:Daiichi Sankyo, Inc.: Current Employment. Slosberg:Daiichi Sankyo, Inc.: Current Employment. Daver:Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Research Funding; Servier: Research Funding; Genentech: Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novimmune: Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Trovagene: Research Funding; Fate Therapeutics: Research Funding; ImmunoGen: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz: Consultancy, Membership on an entity's Board of Directors or advisory committees; Trillium: Consultancy, Membership on an entity's Board of Directors or advisory committees; Syndax: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; KITE: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Andreeff:Daiichi-Sankyo; Jazz Pharmaceuticals; Celgene; Amgen; AstraZeneca; 6 Dimensions Capital: Consultancy; Daiichi-Sankyo; Breast Cancer Research Foundation; CPRIT; NIH/NCI; Amgen; AstraZeneca: Research Funding; Centre for Drug Research & Development; Cancer UK; NCI-CTEP; German Research Council; Leukemia Lymphoma Foundation (LLS); NCI-RDCRN (Rare Disease Clin Network); CLL Founcdation; BioLineRx; SentiBio; Aptose Biosciences, Inc: Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.